Today, Pfizer and BioNTech released interim study results of the late stage phase 3 trial of their vaccine candidate. According to the results, the efficacy of the vaccine is more than 90%, which is much higher than anticipated, as the vaccine has not been under development for a very long time and the efficacy of a normal flu shot is around 40-60%. We think it is important to remember that efficacy is defined as preventing disease (i.e. not getting sick) and not preventing infections. This means that we do not know whether the virus gets less infectious among vaccinated people, or whether the vaccine prevents severe COVID-19 or whether it protects the vulnerable and elderly.
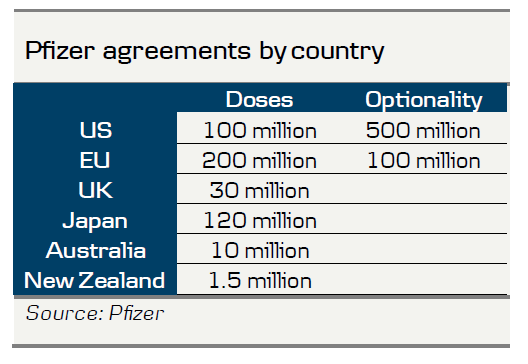
It is also important to remember that the vaccine still needs approval (like the rest of the vaccines currently in phase 3 trials), to be produced and then distributed. People also need to feel safe getting vaccinated and unfortunately as many as 45% of the Americans say they are not willing, according to a recent CNN poll. This will not happen overnight, so we have not fundamentally changed our view that we are heading for a long winter with restrictions in the northern hemisphere, especially in Europe. However, it probably means that the risk that we need to do it all over again next autumn and winter is declining. From a vaccine production and distribution perspective, please notice that the vaccine is based on two shots and that the virus must be maintained at around -75 Celsius degrees, which makes distribution a bit complicated. Pfizer and BioNTech expect to produce 1.3 billion doses next year (so 650 million people can get injected with the vaccine).
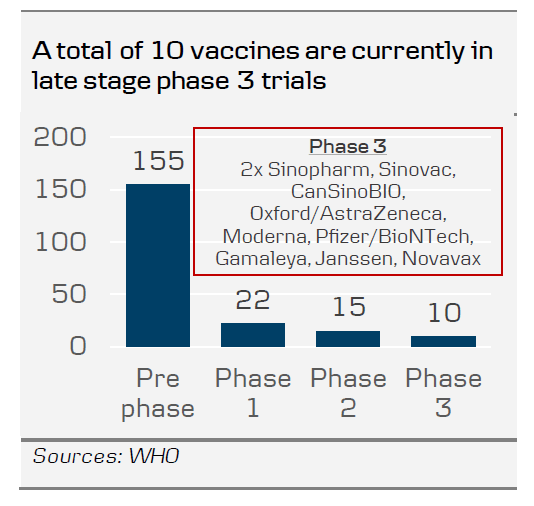
The news from Pfizer could be the turning point for the global economy and financial markets that takes us out of the crisis. The best case scenario from the outset of the virus has been that a vaccine could be ready by the end of the year or early 2021. But even in this scenario most people were expecting a much lower efficiency rate. With 90% efficacy it is much easier to achieve full herd immunity as it lowers the threshold of how many people need to get the vaccine to reach that goal. It thus increases the likelihood that we can return much faster to a world with no restrictions and no risk of new lockdowns. Travelling can return to normal and large gatherings are no longer a risk. Also, remember that we have several other candidates in phase 3 trials right now.
If this scenario turns into the baseline scenario, then a huge cloud of uncertainty would be lifted over the next six months adding a big boost to both consumer as well as business confidence. Pent-up demand on both investment and consumption will likely be unleashed in this scenario and put us on a path of a very robust recovery in 2021. We still need to see confirmation of the results but if we do, it is indeed very good news and it clearly puts upside risks to our growth forecasts. It also underpins risk assets as bankruptcies would go down and profits get a boost
We saw a very significant market reaction after the announcement. Yields rose significantly with both US 10yr Treasury yields and 10yr German government bond yields more than 13bp higher. US 10yr breakeven inflation rates rose by more than 6bp. In the FX space, CHF and JPY were the big losers while NOK and SEK were benefiting. EUR/NOK declined from 10.85 to 10.67 and EUR/SEK declined to below 10.20, the lowest level since early 2019. Oil rose more than 2 dollars to above 42.5 dollars per barrel. Equity markets rose significantly with many of the most COVID-19 exposed industries, such as banks, oil & gas, travel and leisure and autos gaining the most. As we have been arguing for a while, a good and efficient COVID-19 vaccine would be a very important market driver and much more important than the US election, just to mention a recent event. We will not be surp rised if investors continue to trade the “vaccine trade” near-term, especially since we should expect more positive vaccine news before the turn of the year.